Understanding the response of our immune system’s powerful army

Dr Antoine Roquilly and Professor Jose Villadangos
COVID-19 has spread through global populations like a wild fire, consuming whole communities.
However, looking back on the first hundred days of coronavirus transmission in Australia, we have been spared much of this.
Australian government directives enforcing physical distancing and good community compliance have contained the spread of the virus and ‘flattened the curve’ which has saved many lives.
As well as slowing the spread of coronavirus, these restrictions have also inadvertently slowed the spread of another highly contagious and deadly virus – the seasonal flu. This is a very good thing.
Influenza is a serious viral disease that can kill many people in a severe season.
The dynamics of disease
In Australia, the 2019 season saw 312,978 lab-confirmed cases, 3,915 hospitalisations, with 6.3 per cent admitted to Intensive Care Units (ICUs) and 902 deaths.
During flu infection and even after recovery, patients are at high risk of contracting secondary infections and developing fatal pneumonia, but until recently we did not know why.
This is why patients are generally administered antibiotics. But this can encourage multi-resistant strains of bacteria in ICU.
Professor Jose A. Villadangos and his team at Bio21 Institute and the Peter Doherty Institute for Infection and Immunity have been trying to delve deeper, to uncover the underlying cause of this susceptibility to secondary infection after recovery from the primary infection.
They cast their eyes to the immune system.
Working with Dr Antoine Roquilly, a clinician scientist at the University Nantes in France and his group, the team observed what occurs after recovery from severe trauma, flu infection, sepsis (a life-threatening complication of an infection) and a period of time spent in the ICU.
Their new research discovered that recovery from this initial trauma or severe infection leaves an ‘immunological scar’ that reduces the immune system’s capacity to launch protective responses against subsequent infections.
This paralysis can last up to six months, making patients more susceptible to secondary infections like pneumonia.
The COVID-19 treatment trials that 'learn as they go'
We humans have not evolved to cope with the level of inflammatory assault that would send a person to ICU. Modern medicine is the only reason we survive.
But this comes at a cost: the same processes that are normally at work to stop inflammation after the resolution of infection, can ‘overshoot’ in ICU survivors, leaving them immunosuppressed.
Building on the team’s previous work, they found that macrophages (a type of white blood cell of the immune system) in the lungs also show similar immunosuppression after a severe infection or trauma.
Usually macrophages are very good at scavenging and swallowing bacteria, viral particles and debris floating around and in between cells, in a process called ‘phagocytosis’.
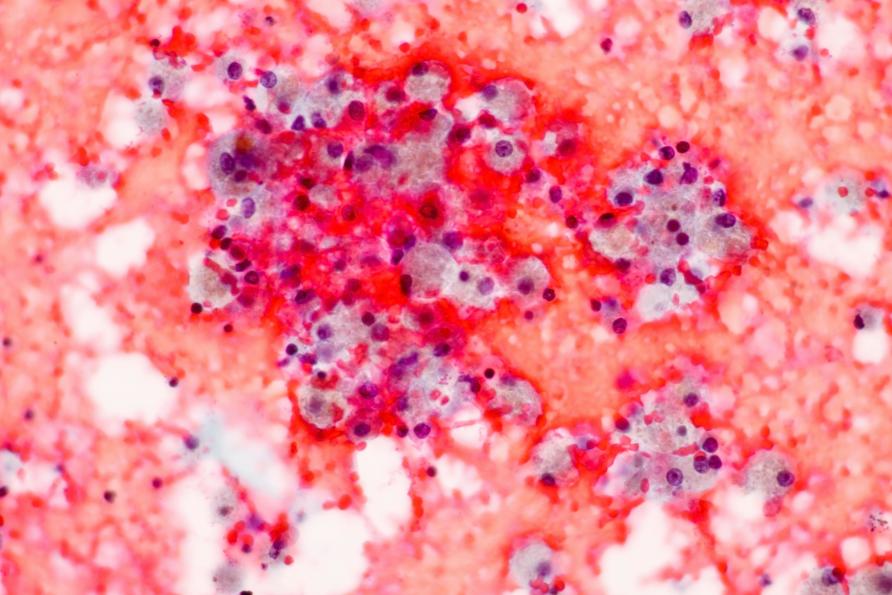
They belong to cells that form the first line of defence of the immune system – our ‘innate immune system.’ They send out alarm signals to activate the immune response and help keep infection at bay before the specific, or ‘adaptive immune system’ kicks in.
But, after a severe lung infection, the team’s research has shown that macrophages are impaired in their ability to phagocytose bacteria.
The new research has also identified the molecular ‘switch’ that results in macrophages being reprogrammed in this way. The program is initiated when the switch is flicked on early in the infection by a ‘signal regulatory protein’ called “Sirp-alpha”.
Flattening the curve to help Australia's hospitals prepare
This establishes an immunosuppressed environment in the lung tissue, affecting all of the macrophages residing in the lungs and has long-lasting effects – up to six months after the initial infection.
It means that once a person recovers from the flu, sepsis or other trauma and leaves ICU, the weeks following their recovery are still an extremely vulnerable time, because their macrophages are unable to effectively respond to bacterial infection.
Having identified this ‘switch’, the team wanted to see whether they could turn it off. The study found that when Sirp-alpha are blocked with antibodies, macrophages’ phagocytic ability to gobble up bacteria was restored.
This changes our thinking about how best to manage patients in ICU.

Rather than treating the patient with antibiotics, that have not been very effective and lead to antibiotic resistant strains emerging, we could be treating the immune system directly by preventing the Sirp-alpha ‘switch’ for immunosuppression from being flicked.
Just a few weeks ago, the world held its breath with the news that the British Prime Minister tested positive to COVID-19 and had been admitted to ICU.
This was a textbook case for the trajectory of the disease when it can go badly for people: after one week of relatively mild symptoms that included fever, the disease took a dramatic dip in the second week, requiring hospitalisation and intensive care.
Protecting our ageing population from COVID-19
Fortunately, Boris Johnson recovered, leaving the ICU without having to be ventilated. He was very lucky.
But for many people around the world, particularly in high risk groups like the elderly, this stage of their disease is fatal.
Why? What is happening here?
Clinical reports are describing a case where, as a result of the viral infection, the immune system has gone into overdrive; a so-called ‘cytokine storm’.
When a virus infects the body, the immune system’s cells strike the alarm. This ‘alarm’ takes the form of chemical messengers called ‘cytokines’ released by the front-line immune cells, the dendritic cells and macrophages.
Cytokines or chemokines are responsible for a range of symptoms of inflammation, that we associate with being sick: raised body temperature, dilating blood vessels and pain.
Inflammation has been classically described in the following terms: dolor (pain), calor (heat), tumor (swelling) and rubor (redness), like a fire burning through the body, drawing attention to the underlying infection.
As a result, like the emergency services to a crash site, immune cells rush to the site of infection. It’s dangerous for the invader, but also potentially dangerous for the body, if this powerful army is not kept in check.
How to take care of yourself if you have COVID-19
In the case of a cytokine storm, rather than retreating when the threat has been eliminated, the immune system’s army keeps fighting. Its toxic weaponry starts to wreak havoc on its own body, destroying cells and tissues and causing organ failure.
A fine balance needs to be struck between reacting quickly and forcefully enough to defeat the infection, but not overreacting.
In COVID-19, we are seeing deaths because a person’s immune system is not controlling the viral infection, it is preparing to respond even more strongly.
This represents the other end of the spectrum to the immunosuppression the team investigated. But research is only starting to understand the triggers and ‘switches’ that govern the behaviour of the front-line cells of the innate immune system, the dendritic cells and macrophages.
And research is only just beginning to decode the cytokine messages they emit that determine the severity of disease progression for bodily trauma and severe infections like flu, sepsis and COVID-19.
Like a pendulum, these messages can swing too far in both directions: a ‘cytokine storm’ is one extreme and immune suppression is the other. But both extremes are deadly.
The team’s research, although it was focussed on flu and sepsis, can provide valuable clues to what may be occurring during a severe case of COVID-19 and recovery afterwards.
Banner: Getty Images
All republished articles must be attributed in the following way and contain links to both the site and original article: “This article was first published on Pursuit. Read the original article.”